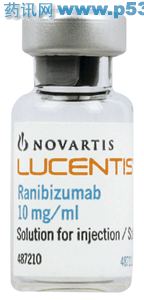
简介:注册证号 S20110085 原注册证号 产品名称(中文) 雷珠单抗注射液 产品名称(英文) Ranibizumab Injections 商品名(中文) 诺适得 商品名(英文) Lucentis 剂型(中文) 注射剂 规格(中文) 10mg/ml,每瓶 ...
关键字:雷珠单抗注射液
注册证号 S20110085
原注册证号
产品名称(中文) 雷珠单抗注射液
产品名称(英文) Ranibizumab Injections
商品名(中文) 诺适得
商品名(英文) Lucentis
剂型(中文) 注射剂
规格(中文) 10mg/ml,每瓶装量0.20ml
注册证号备注
包装规格(中文) 1瓶/盒,内附2个针头和1支注射器
生产厂商(中文)
生产厂商(英文) Novartis Pharma Stein AG
厂商地址(中文)
厂商地址(英文) Schaffhauserstrasse 4332 Stein,Switzerland
厂商国家(中文) 瑞士
厂商国家(英文) Switzerland
分包装批准文号
发证日期 2011-12-31
有效期截止日 2016-12-30
分包装企业名称
分包装企业地址
分包装文号批准日期
分包装文号有效期截止日
产品类别 生物制品
药品本位码 86978679001508
药品本位码备注
公司名称(中文)
公司名称(英文) Novartis Pharma Schweiz AG
地址(中文)
地址(英文) Monbijoustrasse 118 3007 Bern, Switzerland
国家(中文) 瑞士
国家(英文) Switzerland
英文药名: Lucentis (Ranibizumab Injections)
中文药名: 兰尼单抗注射剂
Lucentis (ranibizumab)
"雷珠单抗"治疗糖尿病黄斑水肿再添新证
最新消息报道称,两项评价用于评价雷珠单抗注射液(Lucentis)对糖尿病患者黄斑水肿(DME)作用的3期试验中的第二项已实现了其主要终点。
此项名为RIDE的研究的主要终点显示,经过24个月的治疗后,雷珠单抗注射液组比开始时多阅读视力表上15个字母以上的患者明显多于对照组。药品安全性也与先前的Lucentis3期试验一致。RIDE研究的结果将于2011年5月29日在伦敦召开的欧洲视网膜大会上公布。
早在今年二月,基因泰克公司宣布雷珠单抗注射液对黄斑水肿患者作用的3期试验中的第一项——RISE研究——已实现了主要终点。研究结果显示,接受雷珠单抗注射液治疗的黄斑水肿患者,用药后第七天开始并且维持24个月的快速持久的视力改善。RISE研究结果于3月10日在黄斑学会会议上公布。
黄斑水肿是一种以眼睛视网膜肿胀为特点的病变,是I型或II型糖尿病患者最常见并发症之一,可引起视力模糊、严重的视力丧失和失明。在发达国家,黄斑水肿是人口致盲的最主要原因之一,目前尚没有获美国FDA批准专治药物。RIDE及RISE研究是相同的三期试验,均是为向美国食品及药品管理局申请上市而设计,即将Lucentis用于治疗黄斑水肿的潜在新适应症的支持性研究。
RISE与RIDE这2项研究结果均证实,雷珠单抗注射液治疗的黄斑水肿患者视力能获得明显改善,雷珠单抗注射液有望为临床治疗黄斑水肿的提供了一种潜在新选择
ll Prescribing Information
Lucentis (SAILOR) Dear Healthcare Provider Letter (459K/PDF)
Lucentis? (ranibizumab injection) is a prescription medicine for the treatment of patients with neovascular (wet) age-related macular degeneration (AMD) and macular edema following retinal vein occlusion (RVO).
Status
AMD
In June 2006, the U.S. Food and Drug Administration (FDA) approved Lucentis for the treatment of neovascular wet AMD after a Priority Review (six-month).
The FDA approval of Lucentis in wet AMD is based on data from two large prospective, randomized, masked sham-injection controlled Phase III clinical trials (MARINA and ANCHOR) and one Phase IIIb study (PIER). In the Phase III studies:
?Nearly all patients (approximately 95 percent) treated with Lucentis (0.5 mg) maintained (defined as the loss of less than 15 letters in visual acuity) and up to 40 percent improved (defined as the gain of 15 letters or more in visual acuity) vision at one year, as measured on the Early Treatment of Diabetic Retinopathy (ETDRS) eye chart.
?On average, patients treated with Lucentis in the MARINA study experienced an improvement from baseline of 6.6 letters at two years compared to a loss of 14.9 letters in the sham group. In the ANCHOR study, patients treated with Lucentis, on average, experienced a 10.7 letter gain from baseline at two years compared to a loss of 9.8 letters in the Visudyne? photodynamic therapy (PDT) control group.
?Up to 40 percent of patients treated with Lucentis achieved vision of 20/40 or better.
?The most common eye-related side effects were red eye, eye pain, small specks in vision, the feeling that something is in the eye, and increased tears. The most common non-eye-related side effects were nose and throat infection, headache, and respiratory and urinary tract infections.
In addition to data from the two pivotal studies, data from the Phase I/II FOCUS and Phase IIIb PIER studies were included in the FDA review.
RVO
Lucentis was approved by the FDA for macular edema following RVO on June 22, 2010 after a Priority Review (six-month).
Lucentis was studied in two prospective, randomized, masked sham-injection controlled Phase III clinical trials — BRAVO and CRUISE:
?The BRAVO study assessed the safety and efficacy profile of Lucentis in a total of 397 patients with macular edema following branch-RVO. The percentage of patients in the Lucentis 0.5 study arm who gained 15 or more letters in best-corrected visual acuity (BCVA) from baseline at month six was 61 percent, compared to 29 percent in the sham injection arm. At month six, patients who received 0.5 mg of Lucentis had a mean gain of 18.3 letters compared to 7.3 letters in patients receiving sham injections.
?The CRUISE study assessed the safety and efficacy profile of Lucentis in a total of 392 patients with macular edema following central-RVO. The percentage of patients in the Lucentis 0.5 mg study arm who gained 15 or more letters in BCVA from baseline at month six was 48 percent, compared to 17 percent in the sham injection arm. At month six, patients who received 0.5 mg of Lucentis had a mean gain of 14.9 letters, compared to 0.8 letters for patients receiving sham injections.
?The adverse events reported in BRAVO and CRUISE were similar to previous studies, and no new safety events were observed.
Important Safety Information
?Lucentis? (ranibizumab injection) is a prescription medication given by injection into the eye, and it has side effects. Some Lucentis patients have had detached retinas and serious eye infections. Lucentis should not be used in patients who have an infection in or around the eye or are allergic to Lucentis or any of its ingredients.
?Although not uncommon, Lucentis patients have had eye- and non-eye related blood clots (heart attacks, strokes and death).
?Some patients have had increases in eye pressure within one hour of an injection.
?Serious side effects also include inflammation inside the eye, and rarely, problems related to the injection procedure, such as developing a cataract. These can make a patient’s vision worse.
?The most common side effects to a patient’s eye are increased redness in the whites of the eye, eye pain, small specks in vision, and the feeling that something is in the eye. The most common non-eye-related side effects are nose and throat infections, headache, and respiratory (lung) infections.
?If a patient's eye becomes red, sensitive to light, painful, or has a change in vision, they should call or visit their eye doctor right away.
?Please visit http://www.lucentis.com/ for the Lucentis full prescribing information, and additional important safety information.
Proposed Mechanism of Action
Lucentis is designed to bind and inhibit vascular endothelial growth factor (VEGF)-A, a protein that is believed to play a critical role in the formation of new blood vessels (angiogenesis) and the hyperpermeability (leakiness) of the vessels. In wet AMD, these blood vessels grow under the retina and leak blood and fluid, causing rapid damage to the macula, the portion of the eye responsible for fine, detailed central vision. In RVO, angiogenesis and hyperpermeability can lead to macular edema, the swelling and thickening of the macula, which is the portion of the eye responsible for fine, detailed central vision.
Age-Related Macular Degeneration (AMD)
AMD is a major cause of gradual or sudden, painless, central visual loss in the elderly, brought on by deterioration of the macula and is a leading cause of vision loss in people age 60 and older. There are two forms of AMD — wet and dry. All cases begin as the dry form, but 10 percent to 15 percent progress to the wet form, which can result in sudden and severe central vision loss. More than 1.7 million Americans have the advanced form of this condition.
Retinal Vein Occlusion (RVO)
RVO affects more than 1 million people in the United States and is the second-most common cause of vision loss due to retinal vascular disease, which can develop over a long period of time or occur suddenly. It occurs when the normal blood flow through a retinal vein becomes blocked, causing swelling (edema) and hemorrhages in the retina, which may result in vision loss. RVO is commonly caused by a clot in the retinal vein. Sudden blurring or vision loss in all or part of one eye is common with RVO, although loss of vision can develop over a long period of time. RVO typically affects patients who are more than 50 years old, and the incidence increases with age. People with a history of high blood pressure, hypertension, diabetes and atherosclerosis are at an increased risk for developing RVO.
There are two main types of RVO: branch-RVO, which affects an estimated 887,000 people, and central-RVO, which affects an estimated 265,000 people in the United States. Branch-RVO, which is three times more common than central-RVO, occurs when one of the smaller veins emptying into the main vein of the eye becomes blocked. Usually, the blockage occurs at the site where an artery and a vein cross, and affects only a portion of the retina. Central-RVO, the less common form of RVO, occurs when the main vein of the eye (located at the optic nerve) becomes blocked.
雷珠单抗注射液(诺适得,Ranibizumab Injections,Lucentis)咨询
 爱华网
爱华网